WWW.SOLUTIONFANS.COM - MASTER OF ALL EXAM RUNS
2018 WAEC GCE CHEMISTRY THEORY AND OBJECTIVE ANSWERS – JANUARY/FEBRUARY EXPO
CHEMISTRY OBJ
VERIFIED CHEMISTRY OBJ:
1-10: ACCDDAADAA
11-20: DCDABBDCAA
21-30: CBCBCCCADB
31-40: CCBDCCBACD
41-50: CBDA.C….SOLVE REST OBJ BY URSEF
CHEMISTRY THEORY
(1a)
A compound is the chemical combination of two or more elements.
(1b)
-Presence of large molecule.
-Presence of heat.
-Presence of catalyst..
(1c)
-Nickel, Manganese.
-Platinum, Zinc OR Iron
(1di)
Zn(s) + 2AgNO3—>Zn(NO3)2 + 2Ag(s)
(1dii)
I. Ag is reduced
II. Zn is oxidized
(1e)
16
O – 90% abundance
8
16
O – 10% abundance
8
= The relative atomic mass Ar = (16×90/100) + (18×10/100)
= 1440/100 + 180/100 = 1440+180/100
= 1620/100 = 16.20
(1f)
The ores of iron are:
-Hematitie(Fe2O3)
-Magnetide(Fe3O4)
(1gi)
Biotechnology is the optimism exploitation of biological processes for industrial and other purposes. It is the branch of science that exploits microorganisms fot the production of antibiotics, hormones, etc.
(1gii)
Antibiotics
(1h) An element is a substance which can not be further divided by an ordinary chemical reaction.
(1i)
– fossil fuel for burning
– Biomass burning
– wetlands
(1j)
It is because the dipole induced dipole bonds are stronger in iodine than in bromine and than in chlorine ie there are more electrons in iodine than in bromine and that in chlorine
++++++++++++++++++++++++++
(2a)
It means that the standard electrode potential difference set-up between Zinc and one-molar solution of its ion at 298K
(2b)
(i) Zn²(Aq) + / Zn(s) —- cathode
Cu/ Cu²+(aq) ——- anode
(ii) Zn²+(aq)/Zn(s) // Cu / Cu²+
(iii)
At the cathode —- reduction
At the anode —– Oxidation.
(iv) EΦ = EΦ – E
= 0.34-(-0.76)
= 0.34+0.76
= +1.10V
(2ci)
– Chemical and allied products industries
– sunshine oil and chemical industries
– Eleme petrochemical industries
(2cii) source of raw materials
Nearness to market
Source of amenities
2ciii)
-Pollution of air (surrounding)
-Land excavation and improper with disposal
(2di)
Ag2O(s) +H2O2= 2Ag(s)+ O2(g)+H2O(l)
(2dii)
H2O2 + Cl2 => 2HCl(g) + 2H2)(l)
(2e)
-It is an inert gas
-It is slightly denser than air
-It is slightly soluble in water
++++++++++++++++++++++++++
(3a)
From the passage of steam through a mass of hot coke at
a very high temperature (about 1000 oC).
The water gas is mixed with more steam and passed over iron
(III) oxide (as catalyst) at 450 o C. More hydrogen is produced
and carbon(II) oxide gets oxidized to carbondioxide. ie The carbondioxide is removed by dissolving the above products in water under 30 atm pressure – CO2 dissolves.
The gas is then passed through copper(I) methanoate in
ammonia solution under pressure to absorb any trace of carbon(II) oxide which might be present as impurity if not
removed – the hydrogen produced is pure.
(3aii)
C (s) + H 2 O(g) → CO (g) + H 2(g)
(3aiii)
(i) By the action of a dilute strong acid on metals, such as zinc:
(ii) By reaction amphoteric metals with a strong base, such as sodium hydroxide:
(iii) By electrolysis of water:
(3b)
(i) Decrease in temperature shifts reaction towards product side.
(ii) Increase in pressure shifts reaction towards reactant side.
(iii) Increase in concentration of CO will increase the yield of the product.
(3ci)
Condensation polymerisation is a process whereby many
small monomer molecules join together to form one large polymer, with water, or some other small molecule formed at the same time.
(3cii)
(i) polyamides
(ii) polyacetals
(3ciii)
CH3−COO−CH2−CH3,
(3di)
Allotropy is the existence of a chemical element in two or more forms, which may differ in the arrangement of
atoms in crystalline solids or in the occurrence of molecules that contain different numbers of atoms.
(3dii)
-diamond,
-graphite
(3diii)
-Diamond is used for cutting hard substance.
-Graphite is used to make brake linings, lubricants, and molds in foundries
(3e)
TABLULATE:
(i)
Nitrogen (I) Oide:
-No rection with nitrogen(II)oxide
Oxygen:
-Forms reddish brown fumes with nitrogen(II)oxide
(ii)
Nitrogen (I) Oide:
-Sweet smell
Oxygen:
-Odourless
also don't forget to leave a Reply, we would very MUCH appreciate Your Comments On This Post Below. Thanks!




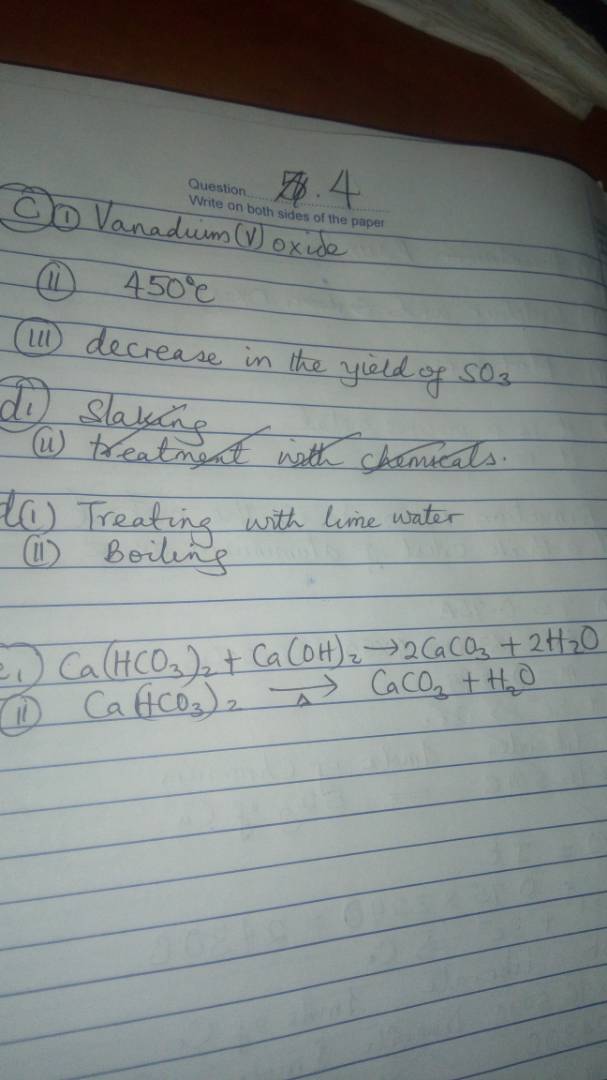

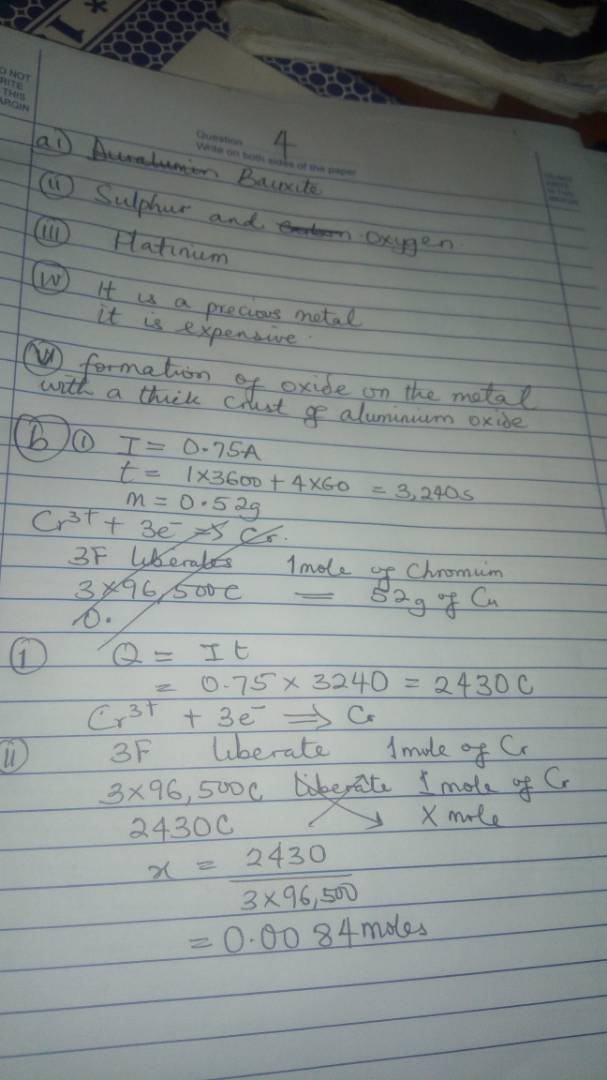


TNKS