WWW.SOLUTIONFANS.COM - MASTER OF ALL EXAM RUNS
ALWAYS SUBSCRIBE IF YOU WANT YOUR ANSWERS ON TIME.
NOTE: WE SETTLE SUBSCRIBERS BEFOR UPDATING HERE…
TELL YOUR FRIENDS ABOUT SOLUTIONFANS NOW!!!!
Chemistry OBJ:
1ABDCBCABAC
11DACBBACCAA
21CBCDCDAAAB
31AABACBABAA
41DBBDCBCCDB
3ai)
H H H H H
| | | | |
H-C-C-C-C-C-C=C-H Heptene
| | | | | |
H H H H H H
3aii)
The sixth member of Allene C7H14 =
7(molecular mass of carbon) + 14(molecular mass of H)
= 7(12) + 14(1)
= 84 + 14 = 98gmol-¹
3aiii)
-Cracking is the breaking down of higher molecules into smaller molecules while reforming is the change in the functional group or activity of the compound.
-In cracking more than one product or component is formed. But in reforming one product can still be gotten.
3bi)
Enthalpy of Neutralization change is the heat change which offers when one mole of H+ from an acid reacts with one mole of OH- from an alkali to form one mole of water.
3bii)
Weigh about 100cm³ of dil HCL(aq) and that of KOH(aq) of equal volume and put each into a glass calorimeter. The temperature of the two Solutions are taken. The mean volume of the temperature of the acid and base taken. Then quickly transfer the alkali to the acid, turn and take the final temperature of the solution. Record the mass of the mixture. Find the temperature difference.
Total heat covered = mass × specific heat capacity × temperature change.
3ci)
I – copper
II – silver
3cii)
Because silver is below copper in the electrochemical series.
3ciii)
Oxidation – anode
Reduction – Cathode
2ai)
Collision theory states that the rate of a reaction depends on the rate of collision of the reactant molecules. Hence effective collision determines the rate of the reaction because it is the collision that leads to the formation of a product.
2aii)
When the rate of collision increases, the rate at which molecules collide with each other will also increase thereby making the kinetic energy of the molecules to also increase. The temperature will also increase because temperature is a measure of average kinetic energy.
2bi)

2bii)
C2H5OH + 3O2 —->2CO2 + 3H2O
1mole of ethanol = 3moles of O2
2.5moles of ethanol will require 3 × 2.5/1 = 7.5moles of O2.
but 1 mole of gas = 22.4dm3
7.5moles = 7.5 × 22.4 = 168dm3 of O2.
2Ci)
Esterification is the formation of an Ester by the reaction between Alkanol and an acid.
2cii)
Two uses of alkanols
i)They are used as solvents for cellulose
ii)They are uses in making perfumes and cosmetics.
iii) They are used for quick drying of paints and nail varnishes.
2ciii)
Sodium Ethanoate
2di)
Tin
2dii )
This is because the galvanized plate is corrosion-resistant. It has a protective coating which prevents further oxidation of the metal.
1a)
Fermentation is the chemical breakdown of a substance by bacteria, yeasts, or other microorganisms, typically involving effervescence and the giving off of heat.
1aii)
disastase
1b)
i)colour change
ii)end point
1c)
1mole
c=n/v=1.0moldm^-3
V=10cm^3=0.01dm^3
n=cv=1.0moldm^-3*0.01dm^3
n=0.01mol of H2SO4
x—>0.01mol of H2SO4
x=56*0.01/1 = 0.56g
mass unreacted = (5-0.56)g=4.44g
1di)
copper (ii) tetraoxsulphate(iv)
1dii)
dyes
1e)
A catalyst increases the rate of a chemical by swinging it’s activation every.
1f)
i) CH3CooH + NH3 —->CH3CooH2 + H2O
ii) Ethanoamide
1g)
i)It doesn’t corrode easily.
ii)it doesn’t react with contain inside.
iiii)It can stand for a long period of time.
1h)
i)Sweet production industries
ii)perfume industries.
1i)
i)evaporation
ii)seeding
1j)
i)it increases the volume water in ocean and seas.
ii)It causes increase in rainfall and also corrode painted surfaces
4ai)
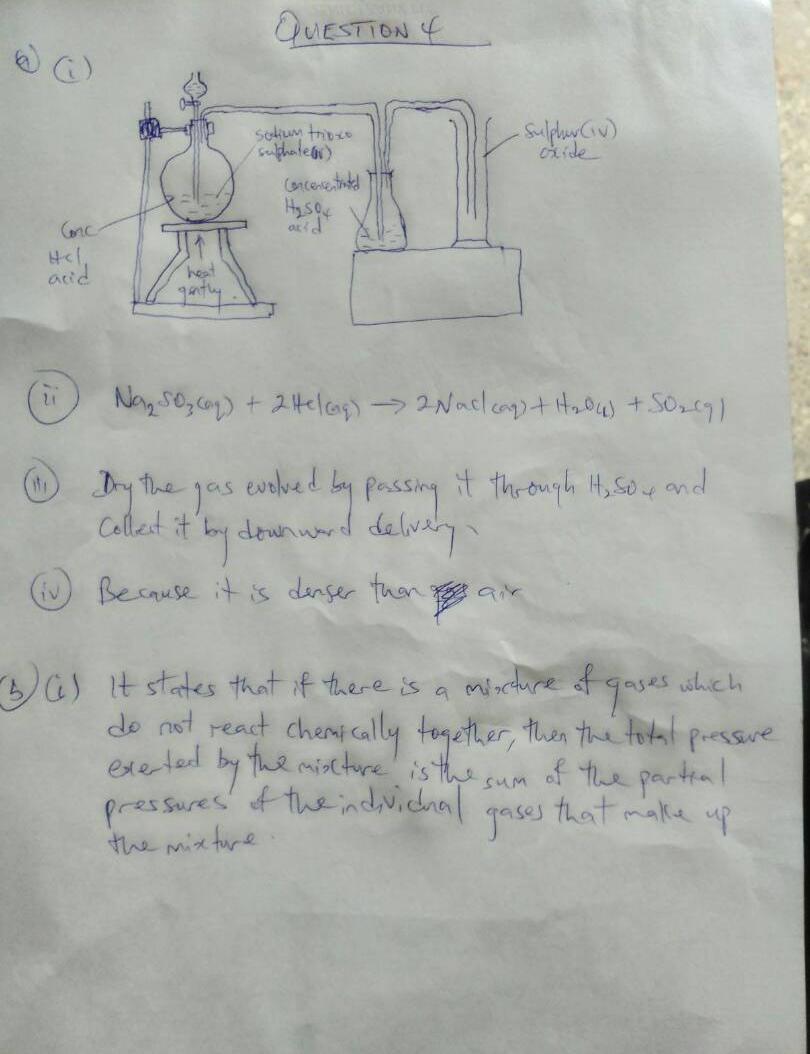
4aii)
Na2SO3(aq) + 2HCl(aq)—–>2Nacl(aq)+H2O(s)+SO2(q)
4aiii)
dry the gas evolved by passing it through H2SO4 and collect it by downward delivery
4aiv)
because it is denser than air
4bi)
Dalton’s law of partial pressure states that in a mixture of gases, which do not react chemically together, a gas will exert a partial pressure, which is the pressure, it will exert if it is kept alone in the vessel.
4bii)
T1=12degree = 285k
P1=690mm Hg
V1=30cm^3
T2=273k
P2=760mmhg
V2=?
Using the general gas law
V1P1/T1 = V2P2/T2
V2=30*690*273/285*760
V2=261cm^3
V2=26cm^3
4ci)
Cl2(q)+H2O(i)——>HCL(aq)+HOCL(aq)
4cii)
litmus turn red because of the presence of the acid HCL
4di)
chlorine bromine iodine/increasing boiling point
4dii)
because water has two(2)ion pairs of electrons as compared to ammonia which has one
COMPLETED
also don't forget to leave a Reply, we would very MUCH appreciate Your Comments On This Post Below. Thanks!


WHAT IS D WEBSITE 4 THE PASSWORD STUFF
http://www.solutionfans.com/home.php
covenant sunday
pls u people should show us d question nw pls
please sir i want to subscribe for E-JAMB but i can’t see the bank account number in the website
Mtn card only
ANITA