WWW.SOLUTIONFANS.COM - MASTER OF ALL EXAM RUNS
Chemistry Practical..
Please click on the image to download it.. Or message 09032305887 on WhatsApp to get it.
Solution centre remains
CHEMISTRY PRACTICAL
Chemistry practical Answers..
You are to answer all.
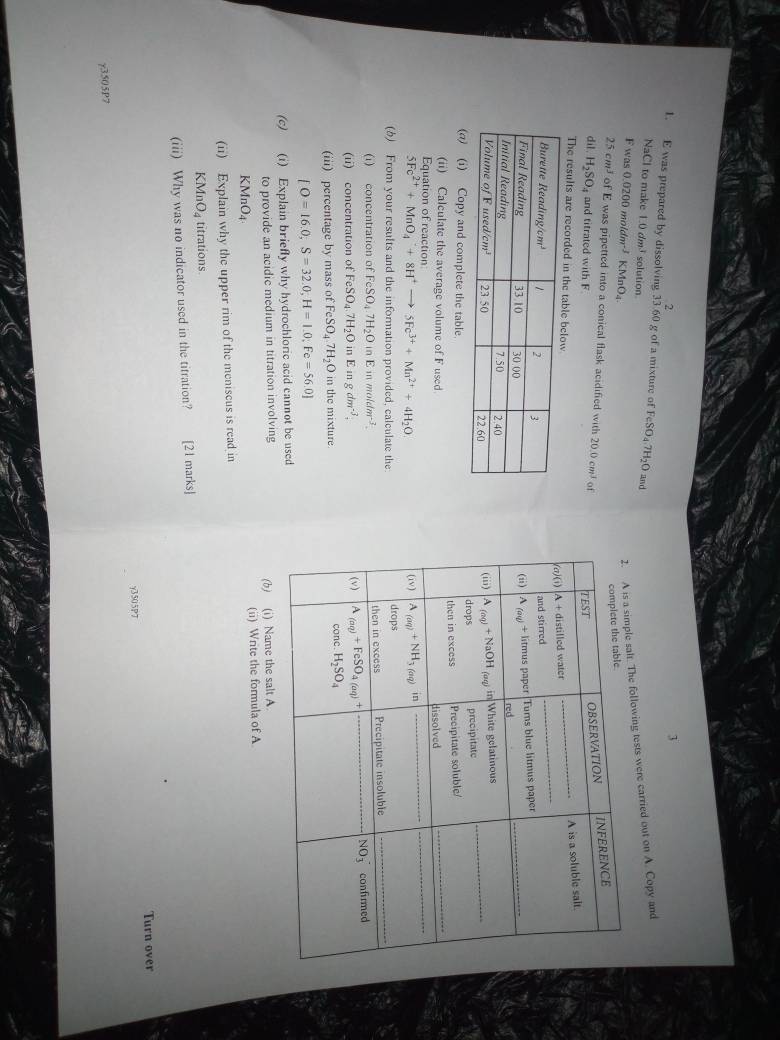
(1ai)
Burette Reading(cm^3)| 1 | 2 | 3 |
Final Reading |33.10|30.00|25.00|
Initial Reading | 9.60|7.50 |2.40 |
Volume of F used (cm^3)|23.50|22.50|22.60.
(1aii)
Average volume of F used
=22.50 + 22.60 / 2
=22.55cm^3
(1bi)
GIVEN:Molar Conc of Kmn04 =0.200mol/dm^2
Volume of Kmn04 used =22.55cm^3
Molar Conc of FeSo4. 7H2O=?
Volume of FeSO4.7H2O=25cm^3
Mole ratio of Kmn04 =1
Mole ratio of FeSO4.7H2O=5
Using,
Molar Conc of KeSO4.7H2O x Volume(FeSo4.7H2O) =n(FeSO4.7H2O)/n(KmnO4)
=molar Conc x 25 / 0.0200 x 22.55 = 5/1
=Molar Conc. x 25=0.0200 x 22.55 x 5
=Molar Conc =0.0200 x 22.55 x 5 / 25
=Molar Conc =0.0902mol/dm^3
(1bii)
Molar mass of FeSO4.7H2O
=56+32+(4*16)+7(18)
56+32+64+126 = 278g/mol
Conc of FeSO4.7H2O in gdm-3 = 0.0900*278
=25.02gdm-3
(1biii)
Mass of FeSO4.H2O = 33.60-25.02
=8.58gdm-3
Percentage of FeSO4.7H2O = (8.58/33.60) * 100
= 25.54%
(1ci)
Hydrochloric acid(HCL) is a mild reducing agent and so it will consume a certain amount of Kmn04 during titration. This will give a slightly higher value of Kmn04 consumption than the actual amount needed by the analyte
(1cii)
As Kmn04 is a dark purple colour solution, it is not possible to take readings from the button of the meniscus on the burette as in usual. Hence, the top, of the meniscus is used(visibility consideration)
(1ciii)
No indicator is used because Kmn04 serves as an indicator
============================================
(2a)
TEST|OBSERVATION|INFERENCE
(2ai)
TEST: A+distilled water and stirred
OBSERVATION: A disolved in water
INFERENCE: A is soluble in salt
(2aii)
TEST: A(aq)+litmus paper
OBSERVATION: Turns blue litmus paper red
INFERENCE: A is an acidiic salt
(2aiii)
TEST: A(aq)+NaOH(aq)- in drops, then in excess
OBSERVATION: -White gelatinous ppt formed.
-Precipitate soluble/dissolved
INFERENCE: Pb^2+ or Zn^2+ or Al^3+ present
Pb^2+ or Zn^2+ or Al^3+ present
(2aiv)
TEST:A(aq)+NH3(aq)- in dropss,
-then in excess
OBSERVATION: -White gelatinous precipitate
-Precipitate insoluble
INFERENCE: -Pb^2+ or Zn^2+ or Al^3+
-Al^3+ present
(2av)
TEST: A(aq)+FeSO4(aq)+conc H2SO4
OBSERVATION: Brown ring forms between the two solutions
INFERENCE: NO3- confirmed
(2bi)
A is aluminium trioxonitrate(v)
(2bii)
Al(NO3)3
(2c)
TEST|OBSERVATION|INFERENCE
(2ci)
TEST: K+NaHCO3(aq)
OBSERVATION: No visible reaction is observed
INFERENCE: NaHCO3(aq) does not show result for alkanols(K)
(2cii)
TEST: K+benedict solution+heat
OBSERVATION: There is no change in the colour of the benedicts solution
INFERENCE: Benedicts solution is not an indicator for alkanols(K)
(2ciii)
TEST: K+KMnO4(aq)
OBSERVATION: KMnO4(aq) solution changes from purple to colourless
INFERENCE: Alkanol is being oxidized by KMnO4
(2civ)
TEST: K+CH3COOH+conc H2SO4
OBSERVATION: pleasant fruity smell is observed
INFERENCE: Esterification reaction has occured
================================================
(3a)
(i) Putsome distilled water in a beaker
(ii) Measure 100cm^3 of 2.00mol/dm^3 of the stock solution by means of a burette and pour all the solution into the beaker containing the distilled water
(iii) Stir the solution
(iv) Transfer the solution into a 250cm^3 volumetric flask by means of a funnel
(v) Ensure that all the solution in the volumetric flask has been transferred by thoroughly rinsing the inside of the beaker with distilled water
(vi) Dilute the solution in the volumetric flask with distilled water until it reaches the 250cm^3 mark
(vii) Shake the solution carefully
(3b)
(i) CH3COONa: It forms basic solution
(ii) Al2(SO4)3: Acidic solution
(iii) KNO3: Neutral solution
(3c)
Acidified potasium tetraoxomanganate(vii) KMnO4 was placed in the burette
INVITE UR CENTER MATES NOW!!!!
=================================
=================================
ALWAYS REFRESH THE PAGE BY UP DRAGGING
NEW CANDIDATES TAKE NOTE: DONT MAKE USE OF ANY ANSWER NOT POSTED HERE OR U WILL HAVE URSEF TO BLAME @ END
=================================
1,733 total views, 1 views today
also don't forget to leave a Reply, we would very MUCH appreciate Your Comments On This Post Below. Thanks!









Today is a good day
Thanks so much … Today Go na b soft work
Am very happy for this ans
Tanx a lot ,, now waiting for them to strt
tans boss.. U guys are d best
Tanx so much..
u guys are the best..
all answer already posted