WWW.SOLUTIONFANS.COM - MASTER OF ALL EXAM RUNS
CHEMISTRTY-Obj
1DDDBCADBBC
11DCDBDDCEDC
21BBDEAAACCA
31BCCBDACDCB
41EAEBEECBAC
51DADCCAABEB
================================
CHEMISTRY-Theory
1a)
I. isotopy is a phenomenon whereby an atom of an element excite different mass number but have the same atomic number.
II. isomerism is existent of two or more compound (known as isomers) with the same molecular formulae but different molecular structure
1aii)
i)Deuterium
ii)tritium’
1bi)
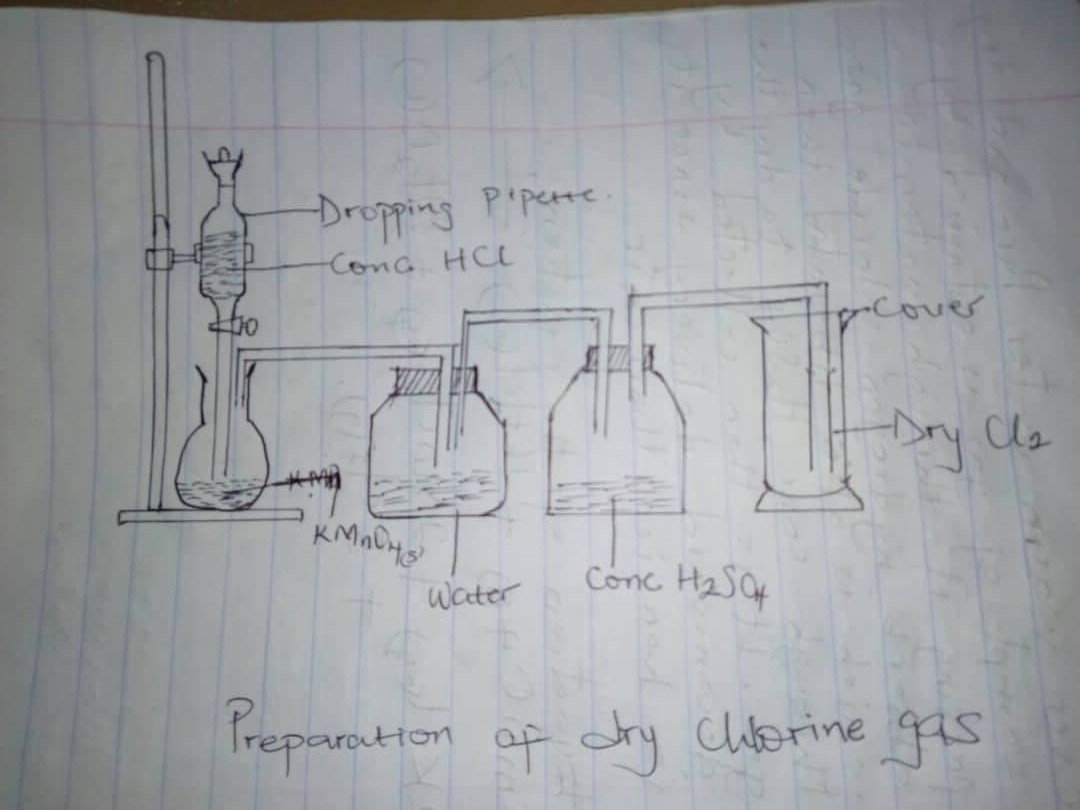
Bottomed flask and set up the apparatus as shown. Add conc HCl gradual into the flask through the thistle funnel pass the gas produced through water and concentrated tetraoxosulphate (iv) and collect it by downward delivery.
2KMnO4(aq)+16HCl—->2MaCl2(aq)+8H2O(s)+5Cl2(aq)
1bii)
i)it is use as a bleaching agent
ii)it is use as disinfectant in the treatment of water
iii)it is use as an oxidizing agent
1ci)
GIVEN
P1=(745-13.5)mmHg =731.5mmHg
T1=(16+273)k=289k
P2=S.P=760mmHg
T2=S.T=273k
V1=40cm^3
using the general gas equation
P1V1/T1=P2V2/T2
V2=731.5*40*273/760*289
V2=36.4cm^3
the volume of the gas at S.T.P is 36.4cm^3
1cii)
i)Nature of reactions
ii)presence of a catalyst
================================
6ai)
NH4^+(aq)+OH^-(aq)+HCl(aq)—> NH4Cl(aq)+H2O(i)
6aii)
the student got the choice of indicator wrong
6aiii)
phenolphthalein is used when a strong base is present. aqueaous ammonia is a weak base
6b)
i)gluclose and fructose
ii)invertase
iii)delivery tube
iv)glucose
6ci)
white phosphorus and red phosphorus
6cii)
I. has no effect of red litmus paper
II. changes damp blue litmus paper pink
III. Reacts directly with alkali to yield a trioxocarbonate(iv)
6di)
GIVEN:
mass of P= 9g
molar mass of P =80g/mol
: Number of mole of P presnt =mass/molar mass
=9/80=0.1125moles
solubility =0.1125/60*100=1.875mol/dm^3
6dii)
C2H5OH(aq) +H2SO4(aq)—-> C2H5HSO4(aq)+H2O(i)
C2H5HSO4(aq)—->C2H4(aq)+H2SO4(aq) ethene
6diii)
Bromine Exists as liquid at room temperature because there is decrease in votality down the group of the halogens due to increasing strength of the van der waal’s force.
================================
3ai)
A homologous series is a family of organic compound which follows a regular structural pattern in which each successive member differ in its molecular formula. By-CH2-GROUP
3aii)
i)solubility
ii)boiling points
iii)densities
3aiii)
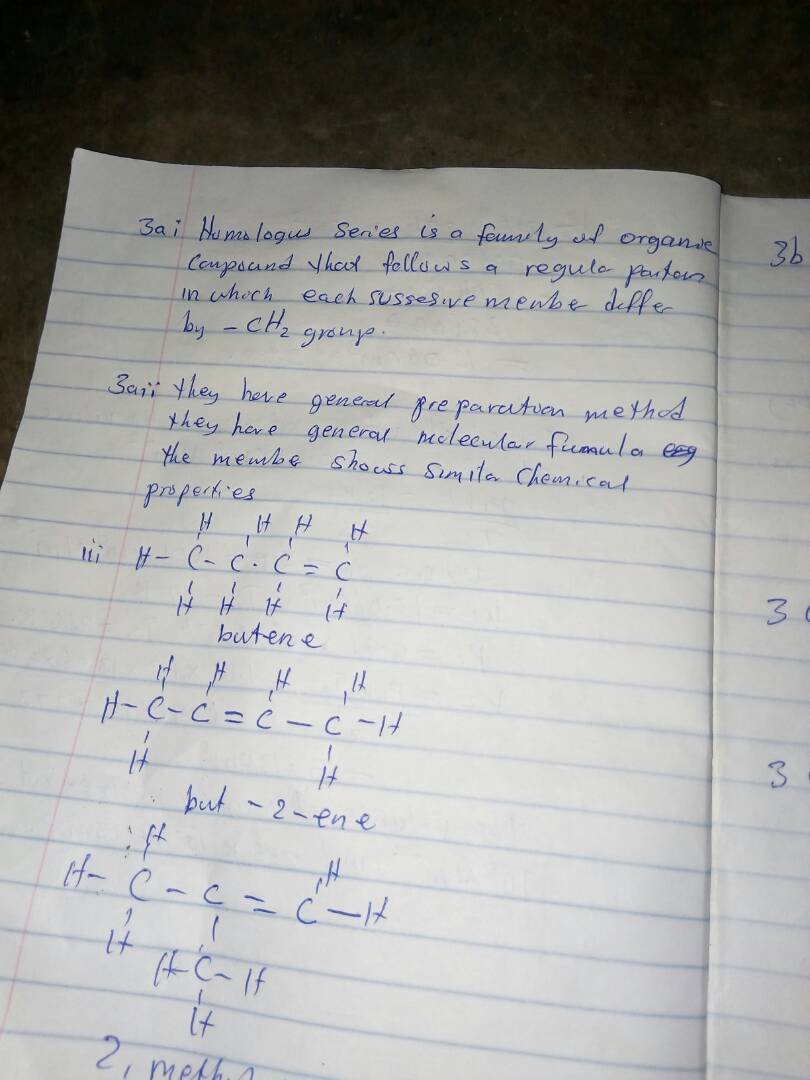 3bi)
3bi)
catalyst are substance which alter the rate of a chemical reaction but not themselves used up in the process.
3bii)
i) they are proteinous in nature
ii)they alter the rate of a chemical reaction
iii)they are not use themselves up in the reaction
3ci)
The solution turn from blue to white as a magnesium displace copper from its salt
3cii)
Mg(aq)+CuSO4(aq)—–> MgSO4(aq)+ Cu(s)
3di)
Equation Reaction:
2Na(s)+O2(q)—–>Na2O2(s)
Number of moles of soduim =mass/molar mass =5.95/23=0.2587moles
reactive ratio of sodium to its oxide is 2:1
therefore number of moles of Na2O2=0.2587/2=0.129moles
mass of oxide
=0.129*[(23*2)+(16*2)]
=0.129*78
=10g
3dii)
i)dissolving an acid on hydride in water
ii)combination of constituent element
3diii)
washing soda (NaCO3. 10H2O)
================================
also don't forget to leave a Reply, we would very MUCH appreciate Your Comments On This Post Below. Thanks!


segun