WWW.SOLUTIONFANS.COM - MASTER OF ALL EXAM RUNS
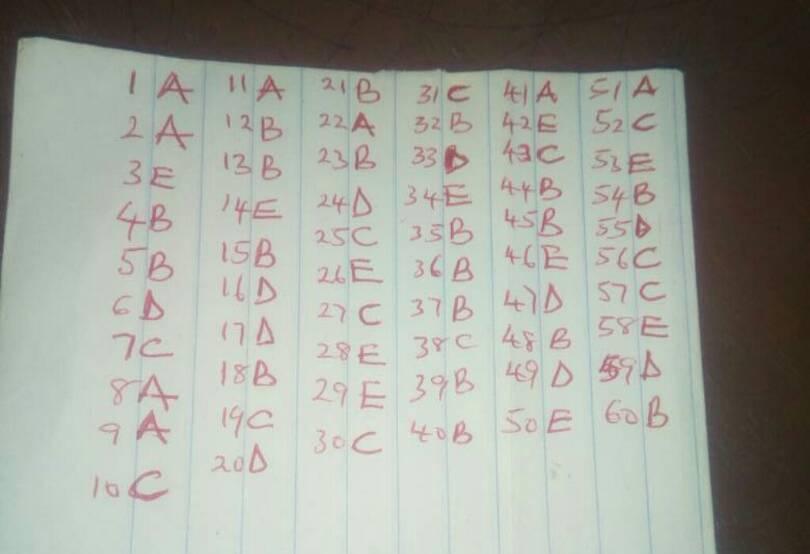
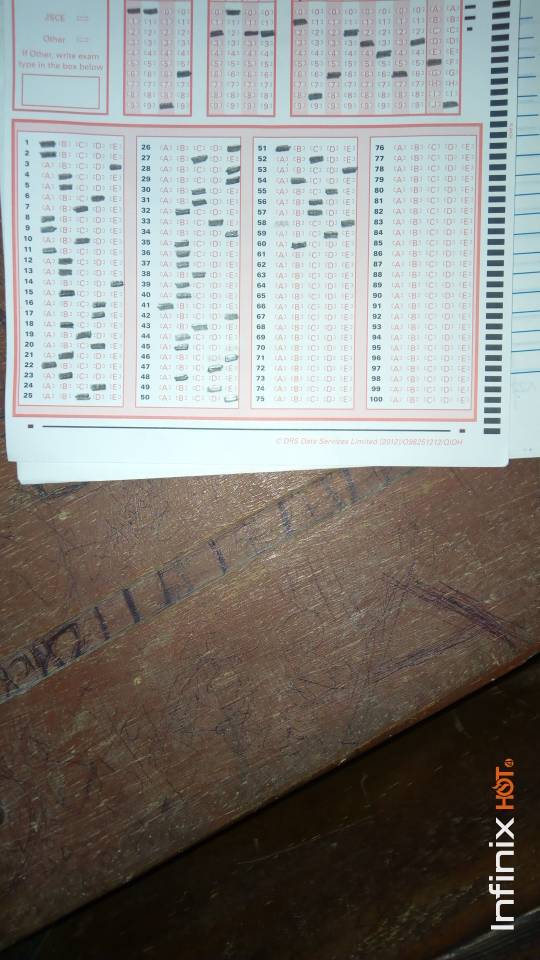
CHEMISTRY OBJ
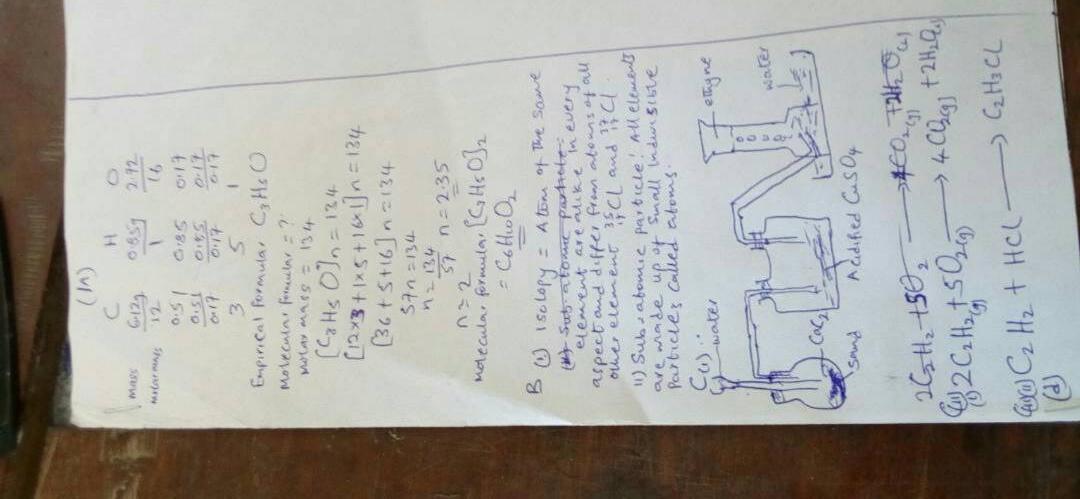
CHEMISTRY THEORY
1)
– Temporary hardness is caused by dissolved calcium hydrogencarbonate
– Permanent hardness is caused by dissolved calcium sulfate.
2bi)
– Temporary Hardness can be Removed By Boiling
– Permanent hardness can be removed by … washing soda (sodium carbonate); ion exchange; the use of polyphosphate water softeners.
2bii)
Advantages:
– Calcium ions in the water are good for children’s teeth and bones.
– Some brewers prefer using hard water for making beer.
2biii)
Disadvantages:
– It is more difficult to form a lather with soap.
– Scum may form in a reaction with soap, wasting the soap.
2ci)
A saturated solution is a chemical solution containing the maximum concentration of a solute dissolved in the solvent.
2ciii)
– Anthracite Coal
– Bituminous coal
– Sub-bituminous coal
2civ)
The anthracite coal: They are the purest forms of coal
2d)
– Zinc is not a transition metal because it forms only Zn2+ ions with all the 3d electrons present.
– Zn not a transition metal, because it forms only Sc3+ ions with n d-electrons
2dii)
Fine Chemicals: This is a chemical compound made in relatively small amounts and in a comparatively pure state.
=============================
3ai)
petroleum gas and petrol->kerosene->diesel->asphalt
3aii)
I-asphalt
II-kerosene
III-petroleum gas and petrol
IV-asphalt
3bi)
i)ethene
ii)propene
3bii)
i)structural isomerism
ii)steveo isomerism
3biii)
19R-1s^2 2s^2 2P^6 3S^2 3P^6 4S^1
10S-1S^2 2S^2 2P^6
24T-1S^2 2S^2 2P^6 3S^2 3P^6 4S^2 3D^4
3biv)
group 6
3ci)
I- AgNO3(aq) and BaCl2
II-BaCl2 and Na2SO4
III-Ca(NO3)2 and H2CO3(aq)
3cii)
i)fitteration
ii)flocculation
iii)disinfection
=============================
5a
i) increasing the surface area
By adding catalyst to the reaction
5iii)0.896dm^3 of CO2 was produced by 4g of caco3
5. If 100g of caco3—–1mole of co2(22.4dm3)
4g——X
X=4*22.4/100
X=0.896dm3
897 total views, 1 views today
also don't forget to leave a Reply, we would very MUCH appreciate Your Comments On This Post Below. Thanks!