WWW.SOLUTIONFANS.COM - MASTER OF ALL EXAM RUNS
(1ai)
Methyl Orange
Reason – Titration of strong acid against base
(1aii)
Yellow
(1aiii)
Hydrochloric acid
(1aiv)2HCL(aq)+ x2Co3 -> 2 x CL(aq) + H20(aq) + CO2(g)
(1bi) Molar mass of HcL = 1+35.5
=36.5gmol^-1
Con of A is moldm^-3 =
Con of A of gdm^3/molar mass in gdm^3
=7.30gdm^-3/36.5gmol^-1
=0.200moldm^-3
(1bii)
CaVa/CbVb=na/nb
Ca=0.200mol/dm^3
Va=26.20cm^3
Cb=?
Vb=25.0cm^3
na=2
nb=1
(0.200*26.20)/(Cb*25.0)=2/1
Cb=(0.200*26.20*1)/(25.0*1)
Cb=5.24/36.5
Cb=0.2098mol/dm^3
(1biii)
Conc. of B in gdm-3/Conc. of B in moldm-3
=10.6gdm-3/0.148 gdm-3
=101.15g
=101gmol-1
(1biv)
Relative atomic mass of X
2x+12+17.8=101
2x=101-29.8
2x=71.2
x=35.6
==================================
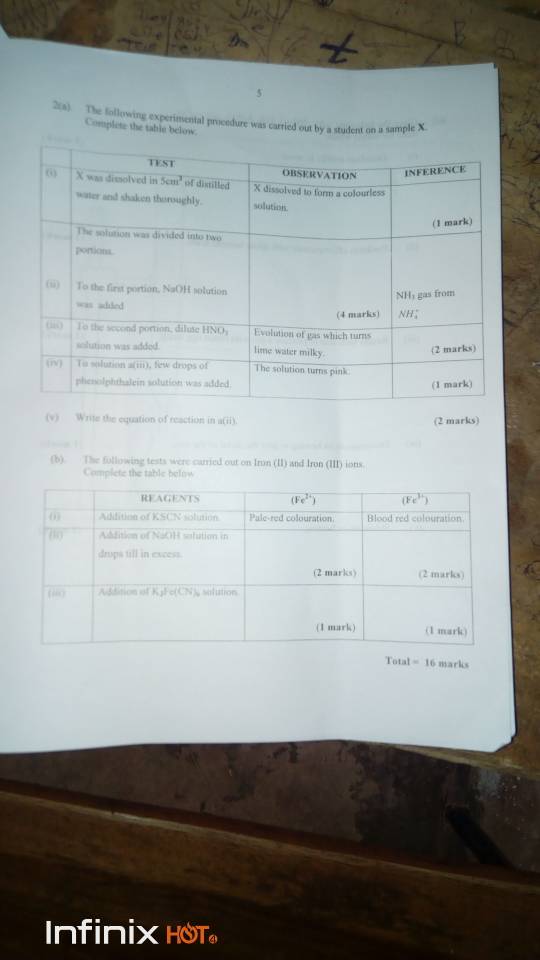
(2ai)
INFERENCE: Sample X is a soluble salt
(2aii)
OBSERVATION: A gas is evolved. The gas turns red moist litmus blue.
It forms white fumed when NaOH solution was added was added
INFERENCE: Alkaline gas
(2aiii)
INFERENCE: CO3 gas is CO3^2-
(2aiv)
INFERENCE: The solution is alkaline
(2av)
(NH4)2CO3(aq)+2NaOH(aq) >> Na2CO3(aq)+2NH3+2H2O(g)
(2bi)
REAGENTS: Adition of KSCN Solution
(Fe2+): Pale-colouration
(Fe3+) Blood red colouration
(2bii)
REAGENT: Addition of NaOH solution in drop till excess
(Fe2+): Dirty green gelatineous precipitaye which is insolube in excess NaOH.
(Fe3+): Reddish brown gelatineous precipitate which is insolube in excess NaOH.
(2biii)
REAGENT: Addition of K2Fe(CN) solution
(Fe2+): It forms a dark blue precipitate
(Fe3+): A deep red colouration
====================================
(3a)
-NH4Cl and AgNo3
-ZnCo3
-FeS
-AgNO3
-NH4Cl
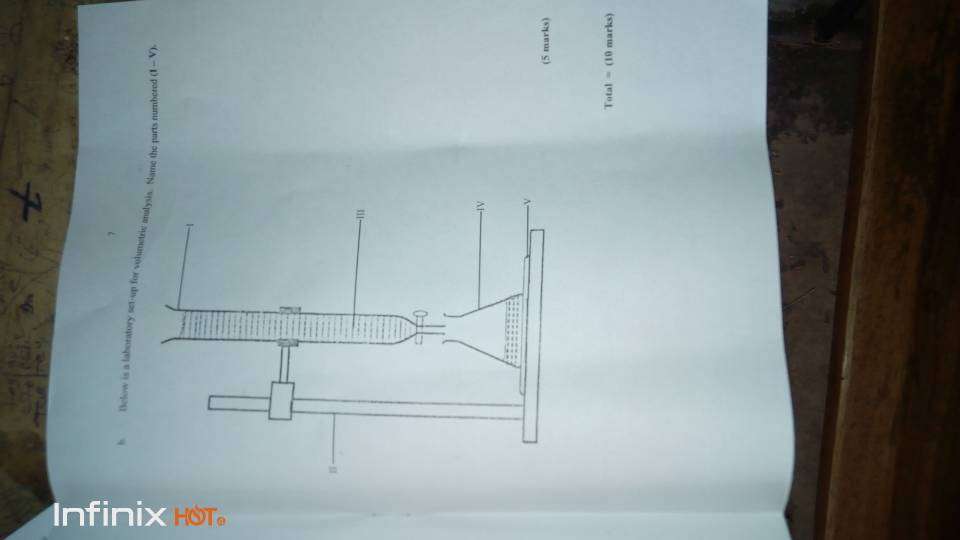
(3b)
-Burrette
-Retort stand
-Solution/Acid
-Conical flask
CHEMISTRY QUESTIONS
726 total views, 1 views today
also don't forget to leave a Reply, we would very MUCH appreciate Your Comments On This Post Below. Thanks!




I am very happy for knowing this site. my today exam was so so sweet . God reward u Sir
Sir I wrote very well
Thank U , Sir plz u will also help me in 2018 jamb. U are good in ans gvn
Thanks so so much. God bless u abundantly